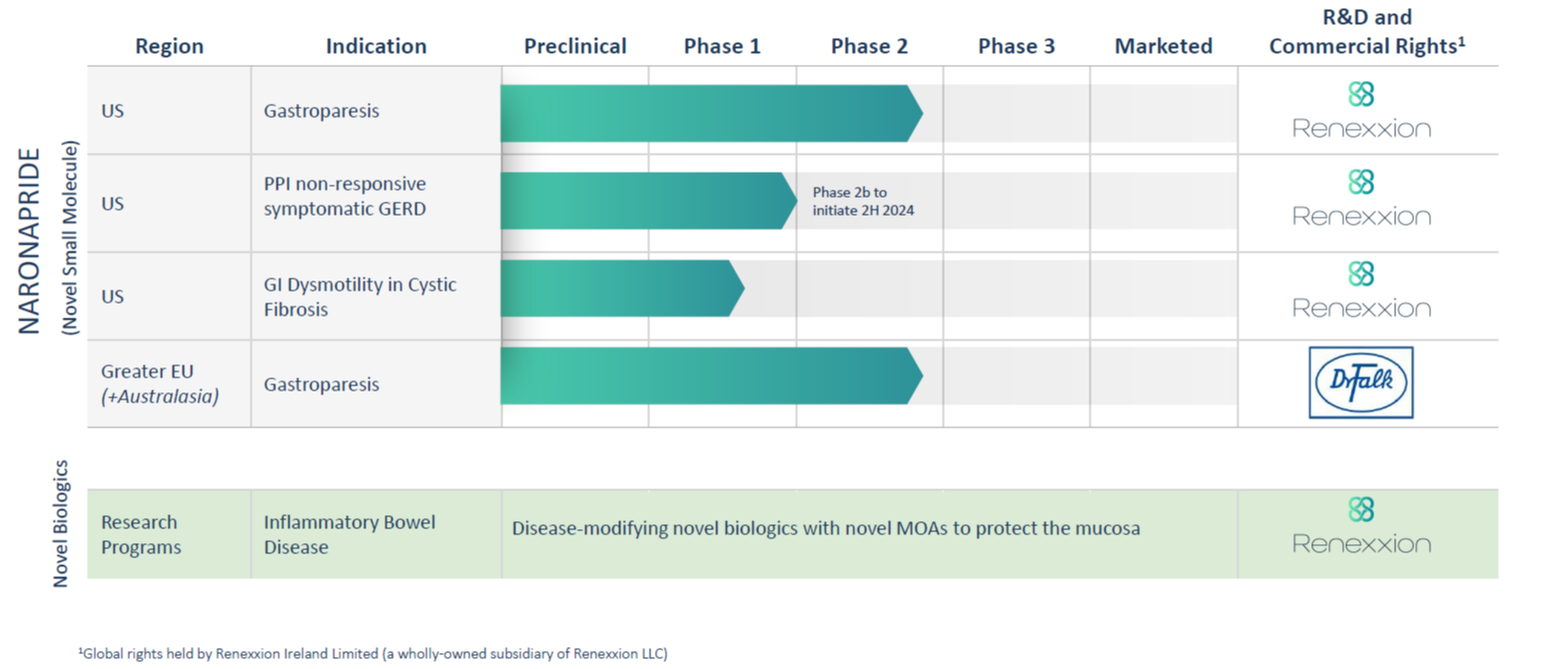
Development Status
Renexxion Ireland is developing its lead drug candidate, naronapride, for use in the treatment of GI motility disorders where a prokinetic is warranted, including gastroparesis and proton-pump inhibitor non-responsive symptomatic gastroesophageal reflux disease (PPI-nrsGERD).
More than 972 subjects have been exposed to naronapride in clinical trials, including four Phase-2 trials, where naronapride showed efficacy signals in both lower and upper GI indications. A Thorough QT (TQT) study testing the cardiac safety of naronapride has also been completed. The results confirmed the cardiac safety profile of naronapride at both therapeutic and supra-therapeutic doses. Naronapride is in late-stage clinical development, with one ongoing Phase 2b trial in gastroparesis and a second Phase 2b trial in PPI-nrsGERD being planned to start.
In 2021, Renexxion Ireland entered into a Licensing and Collaboration Agreement with Dr Falk Pharma GmbH to jointly develop and commercialize naronapride in Greater Europe, Russia, Central Asian Republics and parts of Australasia for an initial indication of gastroparesis. More information about this collaboration can be found here (More...). Dr Falk Pharma is developing naronapride for Gastroparesis and has commenced the Phase 2b MOVE-IT trial in Europe and the United States (More...).
Renexxion Ireland is pursuing PPI non-responsive symptomatic GERD (PPI-nrsGERD) as an indication in the US. GERD affects ~65M people in the US, and up to 40% of patients are non-responsive to treatment with proton pump inhibitors (PPIs). The current treatments do not adequately address the dysmotility elements of GERD and there is a large unmet need for new treatments that improve outcomes and patient quality of life. Studies have demonstrated benefit with prokinetics but there are limited options for patients. We plan to conduct a Phase 2b study to demonstrate the benefits of naronapride in PPI-nrsGERD, when given for 8 weeks, alongside PPIs. The study will evaluate whether there is improvement in heartburn and other symptoms.
More than 972 subjects have been exposed to naronapride in clinical trials, including four Phase-2 trials, where naronapride showed efficacy signals in both lower and upper GI indications. A Thorough QT (TQT) study testing the cardiac safety of naronapride has also been completed. The results confirmed the cardiac safety profile of naronapride at both therapeutic and supra-therapeutic doses. Naronapride is in late-stage clinical development, with one ongoing Phase 2b trial in gastroparesis and a second Phase 2b trial in PPI-nrsGERD being planned to start.
In 2021, Renexxion Ireland entered into a Licensing and Collaboration Agreement with Dr Falk Pharma GmbH to jointly develop and commercialize naronapride in Greater Europe, Russia, Central Asian Republics and parts of Australasia for an initial indication of gastroparesis. More information about this collaboration can be found here (More...). Dr Falk Pharma is developing naronapride for Gastroparesis and has commenced the Phase 2b MOVE-IT trial in Europe and the United States (More...).
Renexxion Ireland is pursuing PPI non-responsive symptomatic GERD (PPI-nrsGERD) as an indication in the US. GERD affects ~65M people in the US, and up to 40% of patients are non-responsive to treatment with proton pump inhibitors (PPIs). The current treatments do not adequately address the dysmotility elements of GERD and there is a large unmet need for new treatments that improve outcomes and patient quality of life. Studies have demonstrated benefit with prokinetics but there are limited options for patients. We plan to conduct a Phase 2b study to demonstrate the benefits of naronapride in PPI-nrsGERD, when given for 8 weeks, alongside PPIs. The study will evaluate whether there is improvement in heartburn and other symptoms.
Pipeline in a Pill: Late-Stage Clinical Development for Multiple Indications in GI
We are committed to ongoing research with a vision of becoming a leading GI biopharmaceutical company globally. We plan to engage with pharmaceutical companies in Greater China and Japan to explore potential future licensing and collaboration partnerships.
References:
El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871-880. doi:10.1136/gutjnl-2012-304269
Machicado J.D., Greer J.B., Yadav D. (2020) Epidemiology of Gastrointestinal Diseases. In: Pitchumoni C., Dharmarajan T. (eds) Geriatric Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-90761-1_7-1
Chen, X. and Wang, F., 2018. Refractory Gastroesophageal Reflux Disease (GERD) Symptoms. In Gastroesophageal Reflux Disease-Theory and Research. IntechOpen.
Fass, R. and Sifrim, D., 2009. Management of heartburn not responding to proton pump inhibitors. Gut, 58(2), pp.295-309.
Rettura, F., Bronzini, F., Campigotto, M., Lambiase, C., Pancetti, A., Berti, G., Marchi, S., de Bortoli, N., Zerbib, F., Savarino, E. and Bellini, M., 2021. Refractory gastroesophageal reflux disease: a management update. Frontiers in Medicine, 8, p.765061.
Kahrilas, P.J., et al., 2012. Regurgitation is less responsive to acid suppression than heartburn in patients with gastroesophageal reflux disease. Clinical Gastroenterology and Hepatology, 10(6), pp.612-619.
Fass, R., 2007. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. Journal of clinical gastroenterology, 41(2), pp.131-137.
Dean BB, Gano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–664.
American Community Survey 2018 (US 18+ population), Lieberman GERD Survey 2010 and 2019, My Total Health 2018, AHRI GERD Survey 2018
References:
El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871-880. doi:10.1136/gutjnl-2012-304269
Machicado J.D., Greer J.B., Yadav D. (2020) Epidemiology of Gastrointestinal Diseases. In: Pitchumoni C., Dharmarajan T. (eds) Geriatric Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-90761-1_7-1
Chen, X. and Wang, F., 2018. Refractory Gastroesophageal Reflux Disease (GERD) Symptoms. In Gastroesophageal Reflux Disease-Theory and Research. IntechOpen.
Fass, R. and Sifrim, D., 2009. Management of heartburn not responding to proton pump inhibitors. Gut, 58(2), pp.295-309.
Rettura, F., Bronzini, F., Campigotto, M., Lambiase, C., Pancetti, A., Berti, G., Marchi, S., de Bortoli, N., Zerbib, F., Savarino, E. and Bellini, M., 2021. Refractory gastroesophageal reflux disease: a management update. Frontiers in Medicine, 8, p.765061.
Kahrilas, P.J., et al., 2012. Regurgitation is less responsive to acid suppression than heartburn in patients with gastroesophageal reflux disease. Clinical Gastroenterology and Hepatology, 10(6), pp.612-619.
Fass, R., 2007. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. Journal of clinical gastroenterology, 41(2), pp.131-137.
Dean BB, Gano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–664.
American Community Survey 2018 (US 18+ population), Lieberman GERD Survey 2010 and 2019, My Total Health 2018, AHRI GERD Survey 2018